Nanosonics AuditPro™ streamlines your organization’s compliance with National standards and evidence-based guidelines. It provides you with real-time risk notifications for easy course-correction and survey-ready ultrasound infection prevention compliance reports.
Meet National standards and evidence-based guideline requirements
Simplify your organization’s ability to meet traceability requirements outlined in your relevant National standards and evidence-based guidelines.1
Nanosonics AuditPro provides automated digital linking of the infection control cycle events, including data captured from trophon®2 devices, to the appropriate patient identifier.
Nanosonics AuditPro has been built to meet HIPAA compliance standards.

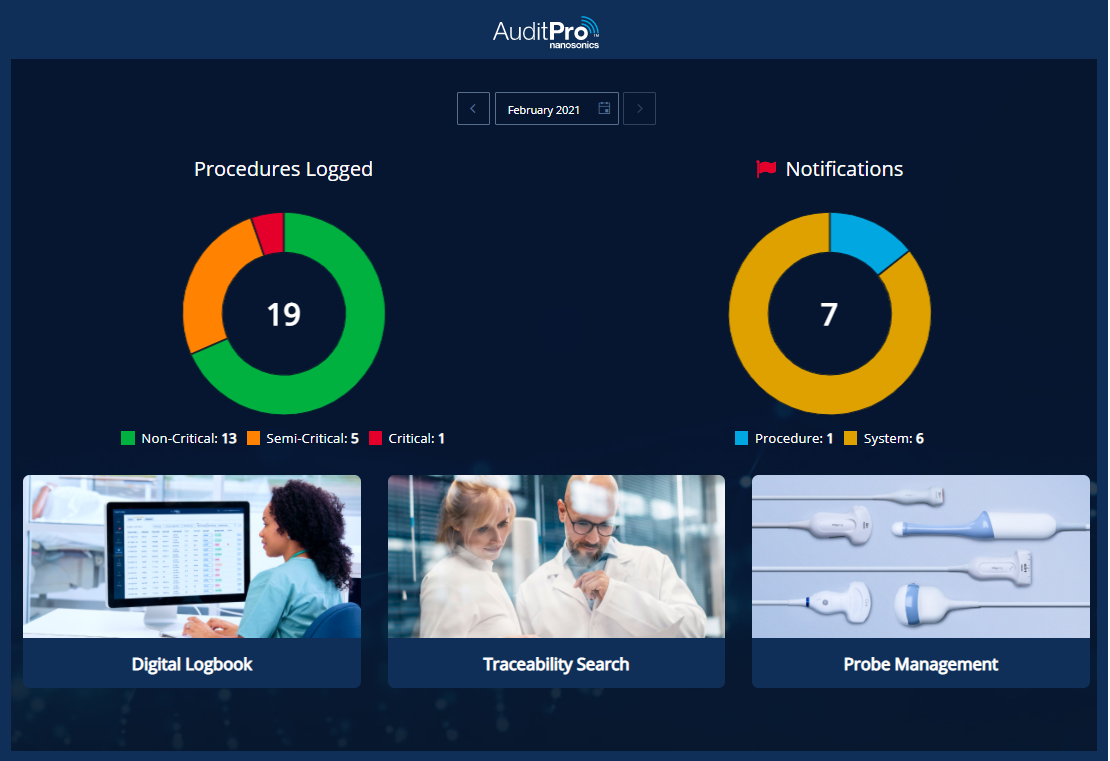
Real-time risk notifications
As an infection preventionist, quality manager or risk officer, you will be able to monitor and course correct compliance over the ultrasound infection prevention workflow.
Real-time notifications are triggered when potential non-compliance events occur providing you an opportunity to remediate by investigating and documenting the event within the system. You can also follow up with training or education on relevant standard operation procedures for your staff as required.
Survey-ready compliance reports
Generate powerful digital logbooks from disinfection data. Search and filter the records easily by any captured data point.
Quickly demonstrate compliance of probes used in procedures with a traceability search by a specific patient identifier.
Print compliance reports, with all relevant critical parameters, and demonstrate a successful disinfection cycle during surveys.

The trophon® family includes trophon® EPR and trophon®2 which share the same core technology of 'sonically activated' hydrogen peroxide.
Book your demo of Nanosonics AuditPro
- Canadian Standards Association (CSA). CAN/CSA-Z314-18 Canadian medical device reprocessing. 2018.

